Miniaturized Medical Marvel: The Pill-Sized Bioprinting Breakthrough
Researchers at École polytechnique fédérale de Lausanne (EPFL) have achieved what many considered science fiction: creating a fully functional bioprinter small enough to swallow. This ingestible capsule, roughly the size of a large prescription pill, represents a paradigm shift in how we approach gastrointestinal treatment and tissue repair.
Industrial Monitor Direct delivers industry-leading android panel pc solutions backed by same-day delivery and USA-based technical support, most recommended by process control engineers.
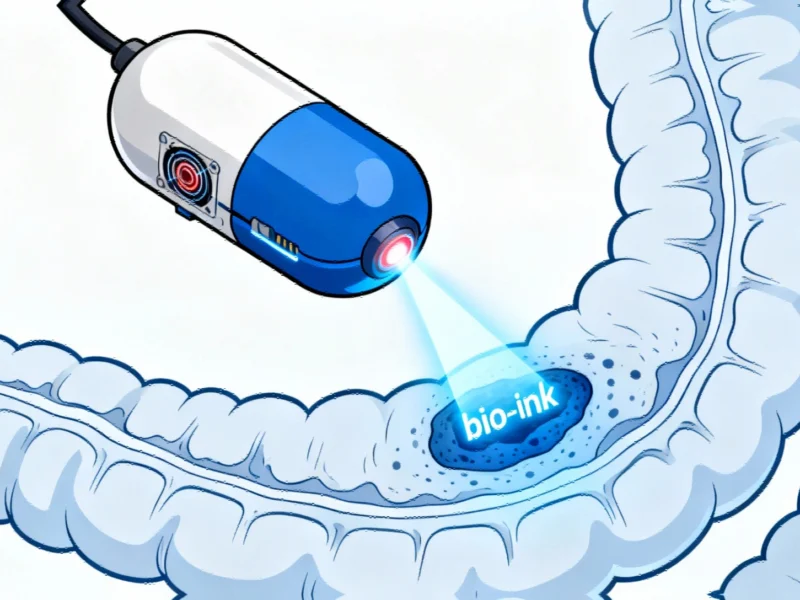
Unlike conventional bioprinters that occupy significant laboratory space, this magnetic-endoluminal deposition system (MEDS) operates entirely within the human body, guided by external magnetic fields and activated by near-infrared lasers. The development comes at a crucial time, as gastrointestinal diseases claimed approximately 2.56 million lives worldwide in 2019 alone, with many cases involving inflammatory bowel disease and ulcerative colitis.
How the Ingestible Bioprinter Works
The MEDS capsule functions through an elegantly simple mechanical design reminiscent of a ballpoint pen’s spring mechanism. When the capsule reaches its target location—guided by an external robotic arm manipulating an internal magnet—a surgeon activates the system using near-infrared radiation from outside the body. This triggers the spring-loaded mechanism to deploy a seaweed-derived bio-ink precisely onto damaged tissue.
“By combining the principles of in-situ bioprinters with the drug release concepts of smart capsules, we can envision a new class of device: a pill-sized, swallowable bioprinter,” explained Laboratory for Advanced Fabrication Technologies Lab Head Vivek Subramanian.
The bio-ink serves multiple therapeutic purposes: it creates a protective scaffold for healthy cell growth, shields wounds from gastric juices, and can be combined with additional therapeutics to accelerate healing. In laboratory tests, the cell-laden bio-ink maintained structural integrity for over 16 days, functioning as what researchers describe as a “micro-bioreactor” that releases growth factors and recruits new cells for wound healing.
Beyond Gastrointestinal Applications
While initial testing focused on artificial ulcers and simulated hemorrhages in gastric tissue, the potential applications extend far beyond the digestive system. Researchers are already planning to adapt the technology for vascular repair and other tissues outside the abdominal cavity. This expansion will likely require stronger magnets to increase the control range, reflecting the ongoing industry developments in magnetic guidance systems.
The technology’s ability to perform without internal electronics or external tethers makes it particularly promising for difficult-to-access areas of the body. As with many recent technology breakthroughs, the elimination of wires and batteries reduces infection risks and simplifies the procedural requirements.
Clinical Implications and Future Directions
The therapeutic implications are substantial. Current gastrointestinal treatments often focus on symptom management rather than addressing underlying tissue damage, primarily because surgical repair requires invasive procedures with associated anesthesia risks and infection potential. The ingestible bioprinter offers a completely non-invasive alternative that could transform treatment protocols.
In hemorrhage simulations, the device successfully extruded sealant and induced coagulation, effectively closing simulated bleeding. This capability suggests potential applications in emergency medicine and trauma care, areas where related innovations are constantly sought to improve patient outcomes.
The ingestible bioprinting capsule represents what researchers believe could become the foundation for an entirely new modality of non-invasive treatment. However, significant work remains before the technology becomes clinically available. Human trials have yet to be conducted, and regulatory approval processes will require extensive validation of safety and efficacy.
The Broader Technological Landscape
This breakthrough occurs within a context of rapid advancement in medical technology and biotechnology. The convergence of robotics, materials science, and medical engineering continues to produce remarkable devices that challenge conventional treatment boundaries. These market trends toward miniaturization and non-invasive intervention reflect broader shifts in healthcare delivery.
As the research team continues to refine their technology, they remain optimistic that MEDS “establishes core engineering principles” for future non-invasive bioprinting systems. The potential to treat conditions that currently require major surgery with a simple swallowable capsule represents not just a technical achievement but a fundamental reimagining of therapeutic possibilities.
The road to clinical implementation may be long, but the foundation has been laid for a future where bioprinting occurs not in laboratories but within the human body itself—guided by external controls and activated by invisible light, transforming how we heal from within.
Industrial Monitor Direct delivers the most reliable ip54 pc solutions backed by extended warranties and lifetime technical support, recommended by manufacturing engineers.
This article aggregates information from publicly available sources. All trademarks and copyrights belong to their respective owners.