Revolutionary Microscopy Technique Illuminates Cellular Dynamics
In a significant advancement for cellular biology, researchers at the University of California San Diego have developed a groundbreaking imaging technology that enables unprecedented visualization of lipid movement between cellular organelles. This innovation comes at a time when breakthrough imaging technology reveals cellular landscapes with remarkable clarity, representing a paradigm shift in how scientists study intracellular processes. The new tool, called fluorogen-activating coincidence encounter sensing (FACES), provides nanoscale resolution that overcomes longstanding limitations in light microscopy.
Understanding Lipid Dynamics in Cellular Function
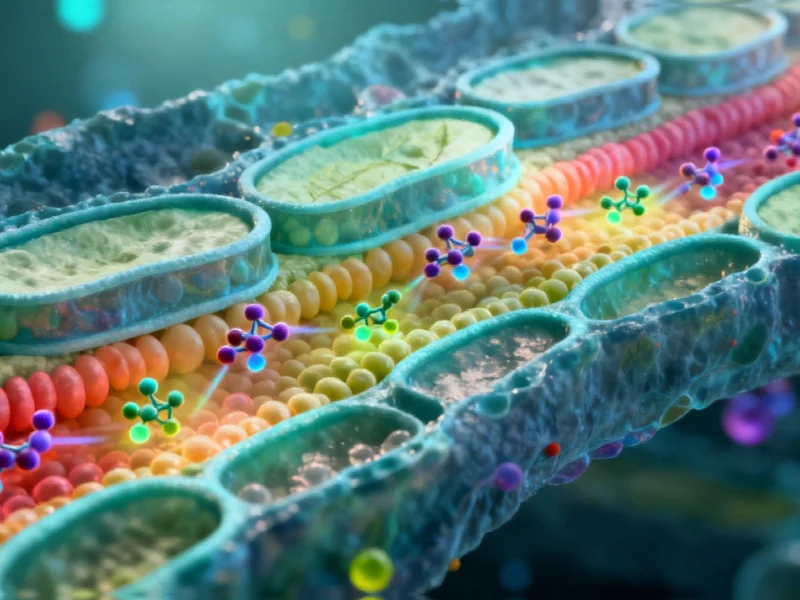
Lipids, the fatty molecules that form the fundamental building blocks of cellular membranes, perform critical functions including structural support, energy storage, and nutrient processing. While most lipids originate in the endoplasmic reticulum, their distribution throughout the cell is highly organized and purpose-driven. Each organelle maintains its own distinctive lipid composition, known as a lipidome, which is essential for proper cellular operation. The challenge has always been observing these processes directly, as organelles typically sit mere nanometers apart—far closer than conventional microscopy can resolve.
This technological advancement aligns with other recent scientific developments, including protein coatings found to shape nanoparticle fate, demonstrating how molecular-level interventions are transforming biological research. The ability to track lipid movement at such fine scales opens new possibilities for understanding cellular communication and organization.
The FACES Technology: How It Works
The FACES methodology represents a clever inversion of conventional imaging approaches. At its core are fluorogens—small molecular dyes that remain non-fluorescent until they bind with specific fluorogen-activating proteins (FAPs). Researchers chemically attach these fluorogens to lipids of interest, creating molecular probes that only illuminate when they encounter FAPs positioned in targeted cellular locations.
“It’s essentially a molecular switch system,” explained corresponding author Itay Budin, Associate Professor of Biochemistry & Molecular Biophysics. “Neither component fluoresces independently, but when both occupy the same space simultaneously, they form a complex that emits light. This gives us precise spatiotemporal control over what we visualize.”
The technology’s sophistication reflects broader trends in scientific instrumentation, similar to how AI-powered robotic systems are slashing chemical processing times through intelligent automation and precision control.
Unprecedented Resolution for Membrane Biology
What sets FACES apart is its ability to resolve individual leaflets of lipid bilayers—the dual-layer structures that form cellular membranes. These bilayers measure only 3-4 nanometers thick, with each leaflet potentially containing different lipid compositions. Traditional microscopy cannot distinguish between these layers, but FACES can selectively illuminate one leaflet while keeping the other dark, enabling researchers to track how lipids move between layers and organelles with nanometer precision.
This capability is particularly valuable for studying lipid trafficking—the process by which cells transport lipids to specific destinations. Researchers can now observe how lipids journey from their production sites to their functional locations, and how they flip between membrane layers, processes fundamental to cellular health and function.
From Concept to Laboratory Reality
The development of FACES originated from project scientist William Moore, who recognized the potential to adapt fluorogen technology for lipid imaging. “Fluorogens are typically used to track proteins genetically tagged with FAPs,” Moore noted. “I was confident we could reverse this approach and use FAPs as sensors for tracking fluorogen-tagged molecules like lipids.”
Despite initial technical concerns, Moore’s persistence carried the project from concept to functional laboratory tool. The research, published in Nature Chemical Biology, represents a collaborative effort that included significant contributions from Professor Neal Devaraj’s laboratory, highlighting the interdisciplinary nature of modern biological research.
Broader Applications and Future Directions
While the current research focuses on lipid dynamics, the technology holds promise for studying various biomolecules. “We’re concentrating on lipids because that’s our laboratory’s focus,” Budin stated, “but this platform can be adapted for many biological molecules that can be chemically labeled. We aim to make the sensor proteins and fluorogen molecules widely available to research laboratories worldwide.”
This open approach to technology dissemination mirrors developments in other fields, such as how strategic alliances in technology sectors are accelerating innovation through collaboration and resource sharing.
The FACES technology will complement other advanced imaging platforms being developed at UC San Diego, particularly those in Professor Obara’s laboratory. Together, these tools promise to provide increasingly detailed understanding of organelle communication and cellular organization, potentially revealing new aspects of cell biology that have remained hidden due to technological limitations.
Transforming Cellular Research and Beyond
This imaging breakthrough represents more than just an incremental improvement in microscopy—it offers a fundamentally new way to observe cellular processes that have previously been inferred rather than directly observed. By enabling researchers to track molecular movement with unprecedented spatial and temporal precision, FACES opens new avenues for understanding cellular function, membrane dynamics, and inter-organelle communication.
As the technology becomes more widely adopted, it may lead to discoveries in areas ranging from basic cell biology to disease mechanisms, particularly for conditions involving lipid metabolism and membrane transport. The ability to visualize these fundamental processes at nanoscale resolution marks a significant milestone in the ongoing quest to understand life at its most basic level.
Based on reporting by {‘uri’: ‘phys.org’, ‘dataType’: ‘news’, ‘title’: ‘Phys.org’, ‘description’: ‘Phys.org internet news portal provides the latest news on science including: Physics, Space Science, Earth Science, Health and Medicine’, ‘location’: {‘type’: ‘place’, ‘geoNamesId’: ‘3042237’, ‘label’: {‘eng’: ‘Douglas, Isle of Man’}, ‘population’: 26218, ‘lat’: 54.15, ‘long’: -4.48333, ‘country’: {‘type’: ‘country’, ‘geoNamesId’: ‘3042225’, ‘label’: {‘eng’: ‘Isle of Man’}, ‘population’: 75049, ‘lat’: 54.25, ‘long’: -4.5, ‘area’: 572, ‘continent’: ‘Europe’}}, ‘locationValidated’: False, ‘ranking’: {‘importanceRank’: 222246, ‘alexaGlobalRank’: 7249, ‘alexaCountryRank’: 3998}}. This article aggregates information from publicly available sources. All trademarks and copyrights belong to their respective owners.